By Minervation,
This is a workshop on clinical research by the Centre of the Cell that can be run by teachers with young people as a learning exercise.
In these activities, pupils learn about how new medicines are developed – from the initial idea, through the science that turns them into treatments, to the clinical research that tests whether they are safe and effective.
 The development of a new medicine, from idea to pharmacy shelf, involves both basic science and clinical research.
The development of a new medicine, from idea to pharmacy shelf, involves both basic science and clinical research.
This workshop takes students through all of the steps that are needed to produce successful new medicines. This includes researching the biology of a disease and how it affects the body. When enough is known about how a disease causes harm, scientists can then develop medicines to intervene. There are many strategies that scientists use when they develop new medicines. Some examples include designing new molecules to block the disease’s interaction with cells, or intervening with a patient’s own immune defences.
In the first activity, Detecting Cancer, pupils need to design a chemical to detect and treat cancer. They need to consider properties of cancer cells, and how a medicine must work to identify and destroy the cancer cells without causing harm to a patient.
The second activity takes students further along the process of developing a new medicine. Students are asked to put several cards in order, describing the steps from research into the biology of disease through the stages of a clinical trial from start to finish.
This game explores several of the factors researchers need to consider when designing new medicines, and demonstrates how important it is to understand how a medicine affects different parts of a patient’s body. Try playing this game several times, trying different options to understand how researchers design medicines and what can go wrong.
 There are many stages in the development of new medicines before they are ready for human use. When scientists find molecules that might have the potential to fight disease, they test them in human cells and in animals to determine whether they are effective and safe. Only then are they ready for testing in humans.
There are many stages in the development of new medicines before they are ready for human use. When scientists find molecules that might have the potential to fight disease, they test them in human cells and in animals to determine whether they are effective and safe. Only then are they ready for testing in humans.
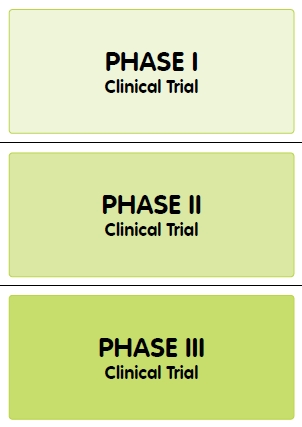
New medicines go through a standard set of steps in clinical trials, called Phases, to test for safety and effectiveness. Phase I tests for the most serious side effects. Phase I trials are often done in healthy volunteers. Phase II trials are usually done in volunteers who have the disease the medicine is targeting, and this Phase determines the most effective dose of a medicine, and how long it stays in the body. Phase III trials are larger in scale, and look for rare side effects which may occur in a larger population.
Most clinical trials follow these Phases, but there are some important variations. Some new medicines, including some cancer treatments, are not tested in healthy volunteers. Several cancer treatments are only tested in terminally ill cancer patient volunteers. This is because these treatments can be toxic to cells. While they might destroy tumours in a cancer patient, these treatments would have side effects that could cause undue harm to healthy volunteers.
In this game, students are asked to design a medicine to treat a new disease, Cellpox. They must put the cards describing the stages in the research and development process for a medicine to treat it, called Poxaway, in the correct order.
To be played in small groups of 4-5
 Materials Needed
Materials NeededWhat do the scientists hope to learn by studying people with Cellpox? How the disease is spread, how it affects systems in the body, possible targets to stop it.
If scientists study Cellpox in people who have it, why do they study it in cells (in vitro) as well? By studying the structure of the Cellpox virus, they can understand more about its shape and properties. It is also a cheaper and faster way to test and exclude ideas that don’t work.
How do scientists find potential molecules to turn into medicines?
They study how a virus or bacteria behaves, and then look for molecules which have shapes or
properties that are likely to interfere with this process.
Why do scientists need to test these new molecules in animals if they appear to work in cells (in
vitro)?
Several compounds can affect other organ systems or processes in the body as well as their intended targets. (For an example of this, try playing the Detecting Cancer game again and choosing to make a fatsoluble medicine with a radioactive tracer. While this medicine may work to identify the cancer, it will also leave radioactive traces that may cause damage to the body, because it stays in the body longer.) New medicines need to be tested in animals, with their complex organ systems, to understand what possible side effects could appear, before they are tested in humans.
Why is Poxaway tested on healthy volunteers first?
The Phase I clinical trials are done on healthy volunteers to ensure that there are no major side effects. Once this is established, Phase II clinical trials are conducted on ill volunteers to test for the optimum dose. It is also important to study how much medicine is needed to be effective, and to measure how long the medicine lasts in the body. This is so the medicine can be given in the correct dose at the appropriate intervals.